Maintaining high customer satisfaction with the purchase of a Nihon Kohden product through its entire life cycle.
To achieve this quality policy, Nihon Kohden sets Group-wide targets and continually strives to ensure quality and enhance customer satisfaction in all processes, from development to production, sales and after-sales services. Our goal is to be a Company that is continually viewed and supported by customers as a trusted partner.
In FY2024, Nihon Kohden conducted three recalls for products manufactured and distributed in Japan, resulting inconvenience to medical professionals.
We are making company-wide efforts to prevent the recurrence and to build up a record of “Zero Recall Days.”
In FY2025, the following initiatives will be implemented as quality targets.
1) We will build a global quality management system to strengthen regulatory compliance and post-marketing monitoring in each country.
In response to the increasing demand for high-quality medical devices at the global level, Nihon Kohden has established its QMS that has strengthened regulatory compliance and post-marketing monitoring in each country or region where its products are sold. We will continue to thoroughly collect and share information on medical device-related laws and regulations in each country/region to shorten the time required for global product registration and ensure the timely supply of products. We will also further upgrade our post-marketing monitoring system and provide feedback on the information obtained for the improvement of internal processes and products to improve the quality of our products and services more rapidly.
2) We will improve customer service as well as software design and manufacturing quality to pursue customer value.
To enhance services for our customers, we will work to improve daily operations, increase efficiency as well as speed, and respond to customer feedback in an appropriate and timely manner. In addition to third-party evaluations of software design, we will continue to work to reduce product failures after shipment by analyzing problems in the product manufacturing process and taking measures to prevent their recurrence.
Nihon Kohden has obtained ISO 9001:2015 certification for its quality management system and ISO 13485:2016 certification for its medical devices and in vitro diagnostics. The Company has also obtained numerous certifications and accreditations including the Medical Device Single Audit Program (MDSAP)* certification. Our Reliability Center has obtained laboratory accreditation in accordance with ISO 17025:2017. In addition, the Company complies with the Medical Device Regulation (MDR) and the In Vitro Diagnostic Medical Device Regulation (IVDR) in Europe.
3) We will work to shorten downtime and reduce failure rates to achieve high product availability.
To increase the utilization rates of products used by our customers, we will continue to work to reduce the failure and re-repair rates. We will also promote the prompt provision of replacement devices in the event of malfunctions and the reduction of delivery times of repair parts and repair times.
4) We will work on human resource development to achieve quality targets and pursue customer value.
To achieve the above quality targets and pursue customer value, we will develop human resources by providing practical training across all Nihon Kohden departments.
* MDSAP is a program to realize a single survey on the compliance and validity of QMS surveys introduced by medical device regulatory authorities in five countries: the U.S., Canada, Brazil, Australia, and Japan.
Medical devices that supports the frontlines of healthcare require exceptionally high levels of quality and safety.
Japan, as well as countries in Europe and North America, have rigorous standards and legislation governing entry into the medical devices market. For example, the U.S., the Food & Drug Administration (FDA) has established the Quality System Regulation (QSR, conventionally known as GMP) for the design and manufacturing processes of medical devices. In Europe, there is the Medical Devices Directive (93/42/EEC), while Canada enforces medical device regulations and quality standards developed by the Canadian Standards Association (CSA).
Nihon Kohden not only complies with these regulations, but was also among the first in the industry to ensure that its systems and practices conform to global standards, enabling the development and manufacture of medical engineering devices with outstanding quality and safety. To demonstrate compliance with these standards, Nihon Kohden has obtained numerous certifications and accreditations from third-party institutions. The Company has also received the Medical Device Single Audit Program (MDSAP) certification ahead of other manufacturers in Japan.
Nihon Kohden continues to strengthen its quality management system and regulatory affairs capabilities to obtain timely approvals in each country and supply products worldwide.
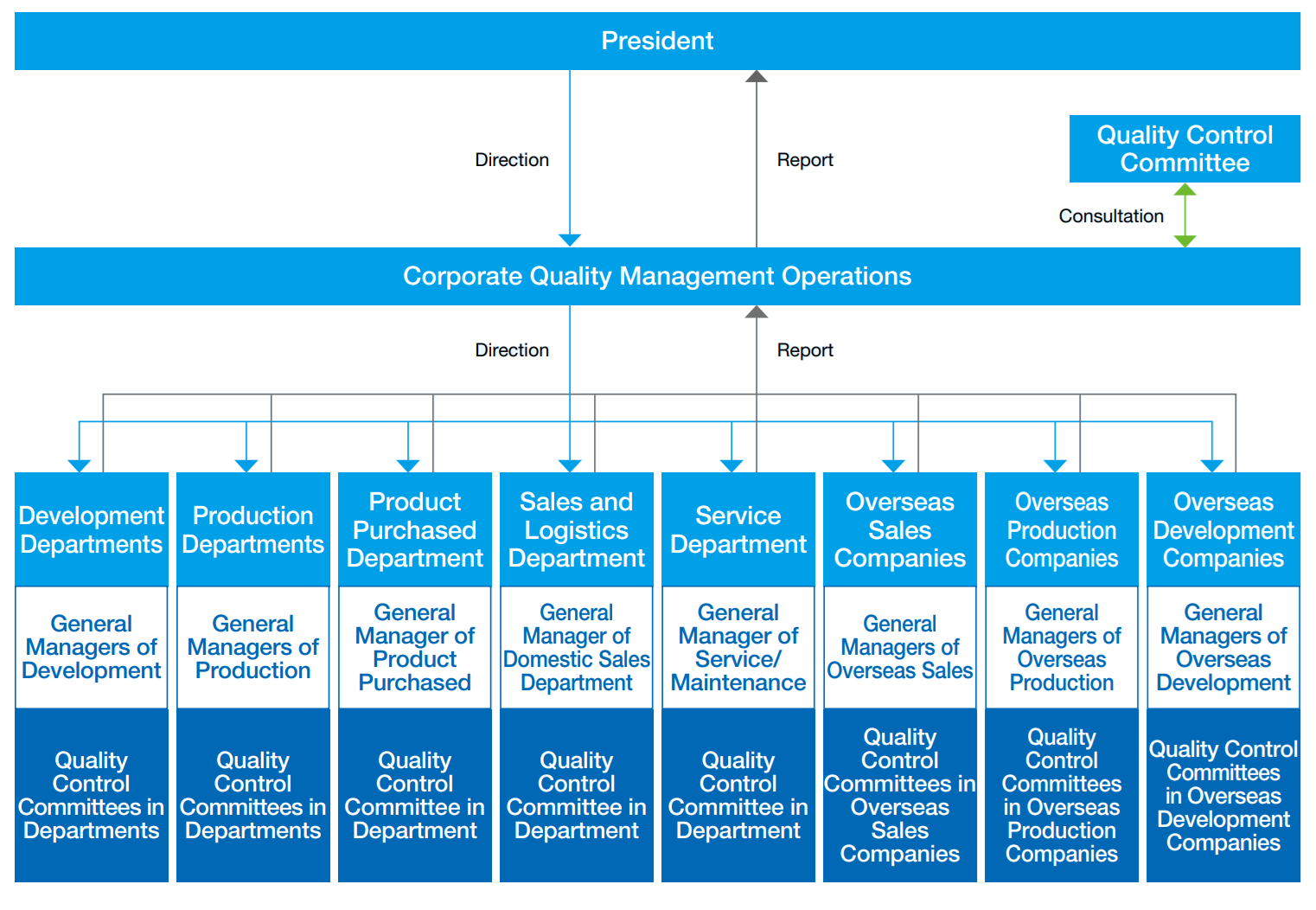
The Comapny has established a system that monitors quality throughout the entire life cycle of its medical equipment - from design, development, and manufacturing to sales, maintenance, and disposal. The results of monitoring at each stage are collected by the Quality System Manager and reported to the President three times a year. Through this system, the Company aims to reduce the quality risk for each product to zero and maintain it there.
Quality Management System
Nihon Kohden has obtained ISO 9001 certification for its quality management system and ISO 13485 certification for its medical devices, and was the first medical devices manufacturer in Japan to receive certification in the CSA’s medical devices category.
- Obtained ISO 9001 certification in January 1995 from the Japan Quality Assurance Organization (JQA). In February 2003, this certification was upgraded to the year 2000 version. In August 2008, the certification body was switched to the British Standards Institute (BSI). This certification was upgraded to the 2008 version in February 2010, and the 2015 version in December 2016.
- Obtained certification in accordance with the EU Medical Devices Directive (MDD) in July 1996 from BSI.
- Obtained ISO 13485 certification (including certification under Canada’s medical device regulations) in February 2003 from BSI. ISO 13485 certification was obtained from BSI in August 2008 and upgraded to the 2016 version in December 2016.
- Obtained MDSAP certification (Medical Device Single Audit Program) in October 2018 from BSI.
- Obtained certification in accordance with the EU Medical Device Regulation (MDR) in April 2022 from BSI.
- Obtained certification in accordance with the EU In Vitro Diagnostic Medical Device Regulation (IVDR) in August 2022 from TÜV SÜD Product Service GmbH.
In addition, all overseas production sites of the Nihon Kohden Groups develop and manufacture products in accordance with their quality management systems that are commensurate with the risks of each product and comply with the law and regulatory requirements of each country. All overseas production sites of the Company Group have obtained ISO 13485 certification, and strive to maintain and improve the quality of their products and services through regular audits by a third-party organization.
|
ISO 9001Quality Management System |
ISO 13485Medical Device Sector Standard |
|
|
Certification Date |
Certification Date |
|
|
Nihon Kohden Corporation* |
January 1995 |
February 2003 |
|
Nippon Bio-Test Laboratories Inc. |
May 2014 |
- |
|
Nihon Kohden America, LLC |
- |
March 2016 |
|
Defibtech, LLC |
- |
February 2004 |
|
Nihon Kohden OrangeMed, LLC |
- |
June 2019 |
|
Neurotronics, LLC |
- |
October 2009 |
|
Nihon Kohden Digital Health Solutions, LLC |
- |
June 2022 |
|
Ad-Tech Medical Instrument Corporation |
July 1999 |
September 2000 |
|
Nihon Kohden Europe GmbH |
November 1995 |
November 1995 |
|
Nihon Kohden Firenze S.r.l. |
December 1995 |
December 1999 |
|
Software Team Srl |
July 2013 |
July 2013 |
|
Shanghai Kohden Medical Electronic Instrument Corp. |
December 1995 |
December 2003 |
|
Nihon Kohden Malaysia Sdn. Bhd. |
- |
April 2015 |
|
Nihon Kohden India Pvt. Ltd. |
August 2018 |
August 2018 |
|
Nihon Kohden Middle East FZE |
July 2020 |
June 2020 |
- Including Nihon Kohden Tomioka in the scope of certification.
Nihon Kohden focuses a great deal on product evaluations in order to develop medical devices that have high degrees of quality and safety. Independent from development, manufacturing and sales departments, our Reliability Center serves as an organization that carries out appropriate product evaluations and consists of iNARTE (The International Association for Radio, Telecommunications and Electromagnetics) - EMC (Electromagnetic Compatibility) and PS (Product Safety) engineers. The Reliability Center is accredited as a third party laboratory that complies with ISO/IEC 17025.
- Obtained laboratory accreditation in the field of electromedical apparatuses in accordance with ISO/IEC 17025 (JISQ 17025) from the Japan Accreditation Board (JAB) in October 2007.
- Obtained laboratory accreditation in field of EMC in accordance with ISO/IEC 17025 from the NVLAP~{*} in March 2009 (which terminated at the end of March 2019) and JAB in March 2019.
*NVLAP: National Voluntary Laboratory Accreditation Program run by the National Institute of Standards and Technology (NIST)













