Nihon Kohden is highly cognizant of the fact that medical devices directly involve human lives. To ensure that its products being used by customers and patients are safe and secure, Nihon Kohden makes daily efforts to improve the quality of its products. However, in the unlikely event a defect is found in one of our products after shipment that poses even a slight risk to the health of users, we have systems in place that enables us to carry out an immediate response and guarantee the safety and security of our customers and patients.
Nihon Kohden compiles a host of information on safety, including defects discovered in the design and production process, safety information obtained from sources overseas, and customer complaints, among other sources. In this way, we are constantly monitoring for possible health risks posed to customers and patients.
Whenever new information on safety is obtained, the Chief Marketing Officer, Quality Assurance Officer and Safety Management Officer immediately convene a meeting to examine ways to address products that have already been shipped and to determine if a product recall or modification is necessary.
If a recall or modification is required, the health threat is categorized either as Class I, Class II or Class III following Japan’s Pharmaceuticals and Medical Devices Act, and Nihon Kohden as well as the Pharmaceuticals and Medical Devices Agency publish pertinent information on their websites. Immediately thereafter the Nihon Kohden Group’s sales and service staff contact customers directly.
Nihon Kohden strives to ensure safety even after product delivery by supporting customers’ safety management and the dissemination of medical technologies.
The Company has assigned specialized Medical Equipment Safety Advisers (MESA) throughout Japan, who have received accreditation as Medical Device Information Communicators (MDIC)*, to hold training sessions on the proper use of its products. In addition to in-person training sessions, we also provide online-based training and audio-narrated slide materials. Through the training materials, we delivered essential safety information to many medical facilities and healthcare professionals in FY2024.
FY2024 Results
- 1,535 sessions
(In-person training: 1,239 times, providing materials (including video files): 284 times, online-based training sessions: 12 times) - 29,425 participants
(In-person training: 23,537 participants, non-in-person training: 5,888 participants) - 691 facilities
Main workshop topics
- Safety workshops for use of patient monitors, defibrillators, ventilators and AEDs
- Safety workshops for electrical safety, safety management of medical devices, and alarm report for patient monitors
*About the MDIC certification program
To improve the quality and safety and ensure correct usage of medical devices, the MDIC certification program is run by the Japan Society of Medical Instrumentation (JSMI) and accredits and develops personnel who can contribute to patient safety and the improvement of healthcare quality, by collecting and providing information on medical incidents and defects and sharing knowledge and technical information necessary for correct usage and maintenance of medical devices between medical safety managers, medical equipment safety managers, medical device users (physicians, nurses, and clinical engineers, etc.) and medical device marketing companies, including manufacturers, marketers and licensors, and repair outlets. To become a Medical Device Information Communicator, a person must take the MDIC seminar organized by the JSMI and pass the certification exam.
As more and more locations install AEDs, there have been situations where an AED was not readily available in good working order because the battery needed replacing or the electrode pads had passed their expiration date.
Committed to helping customers with daily maintenance to ensure their AED can be used at any time
Given this commitment, Nihon Kohden developed the AED Linkage AED remote monitoring system. This system helps support daily maintenance by notifying customers of issues with the AED, or the expiration date of the AED’s electrode pads and batteries, using an email generated based on information gathered from a self-test performed by the AED unit and sent to a server via a remote monitoring terminal. Nihon Kohden is dedicated to providing an environment where all of its customers can be able to readily use an AED with peace of mind.
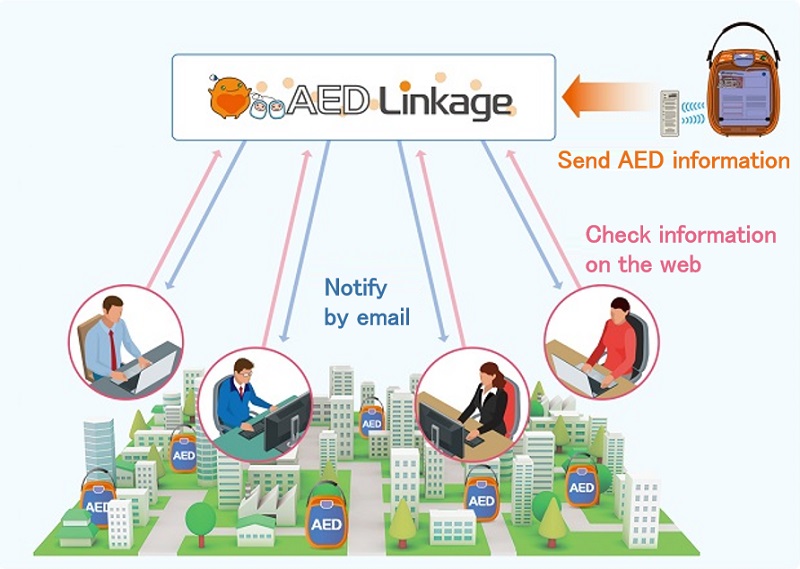
- 拡大
- AED Linkage - AED Remote Monitoring System
Nihon Kohden’s Customer Center (Call Center) provides 24/7 support to assist customers with resolving any issues or failures related to our products and IT systems.
We have dedicated support lines for each product category. Our operators receives product-specific training and our technical support teams assign dedicated engineers for each product category. These efforts enable us to respond promptly and accurately to customer inquiries.

Nihon Kohden organizes vital signs seminars and seminars co-sponsored with academic societies and for all physicians and other healthcare professionals as part of its efforts to ensure the latest, most advanced medical devices are used and understood correctly on frontlines of medicine.
In July 2004, Automated External Defibrillators (AED) were approved for uses by the general public in Japan and since then many public facilities have installed AED units onsite.
Nihon Kohden organizes classes on CPR and AED to ensure that more of the general public are able to use an AED if need be.
Number of AED lectures and participants in Japan in FY2024:
-
613 lectures
(Public training session: 40 lectures, On-site training session: 458 lectures, Online training session: 115 lectures) -
6,797 people
(Public training session: 334 people, On-site training session: 5,459 people, Online training session: 1,004 people)
Nihon Kohden regularly conducts customer satisfaction surveys to continually improve the quality of its products and services. Feedback and opinions from these surveys are shared with development, production, sales and service departments.
Nihon Kohden had set one of the material issues in the Three-year Business Plan, BEACON 2030 Phase II, as “Pursue the highest level of quality in the world,” and established the Net Promoter Score (NPS®)* as a KPI. The improvement of NPS® has been a KPI since the period of the previous Three-year Business Plan, and the NPS® survey was conducted for the fourth time in FY2024. The overall NPS® survey score was -5.3 points in FY2021, -4.0 points in FY2022, and temporarily declined to -8.4 points in FY2023, but improved to -4.1 points in FY2024. In the evaluation of individual scores related to “Corporate Image and Contribution to Customer Value,” the score for “Product cost effectiveness” was improved. On the other hand, the score for “Technology development capability” continued to need improvement. Survey items are reviewed from time to time in light of changes in social demands and customer needs. Beginning with the FY2024 survey, we have added “Environmental considerations such as resource and energy conservation” and “Product design and appearance” to the survey items in an effort to more accurately grasp customer opinions and requests.
Through the NPS® survey, we will continue to further enhance the points that have been highly evaluated by our customers and actively improve the points that need improvement. As a partner to medical professionals, we will continue to be a company that works together with them to solve issues facing the medical field.
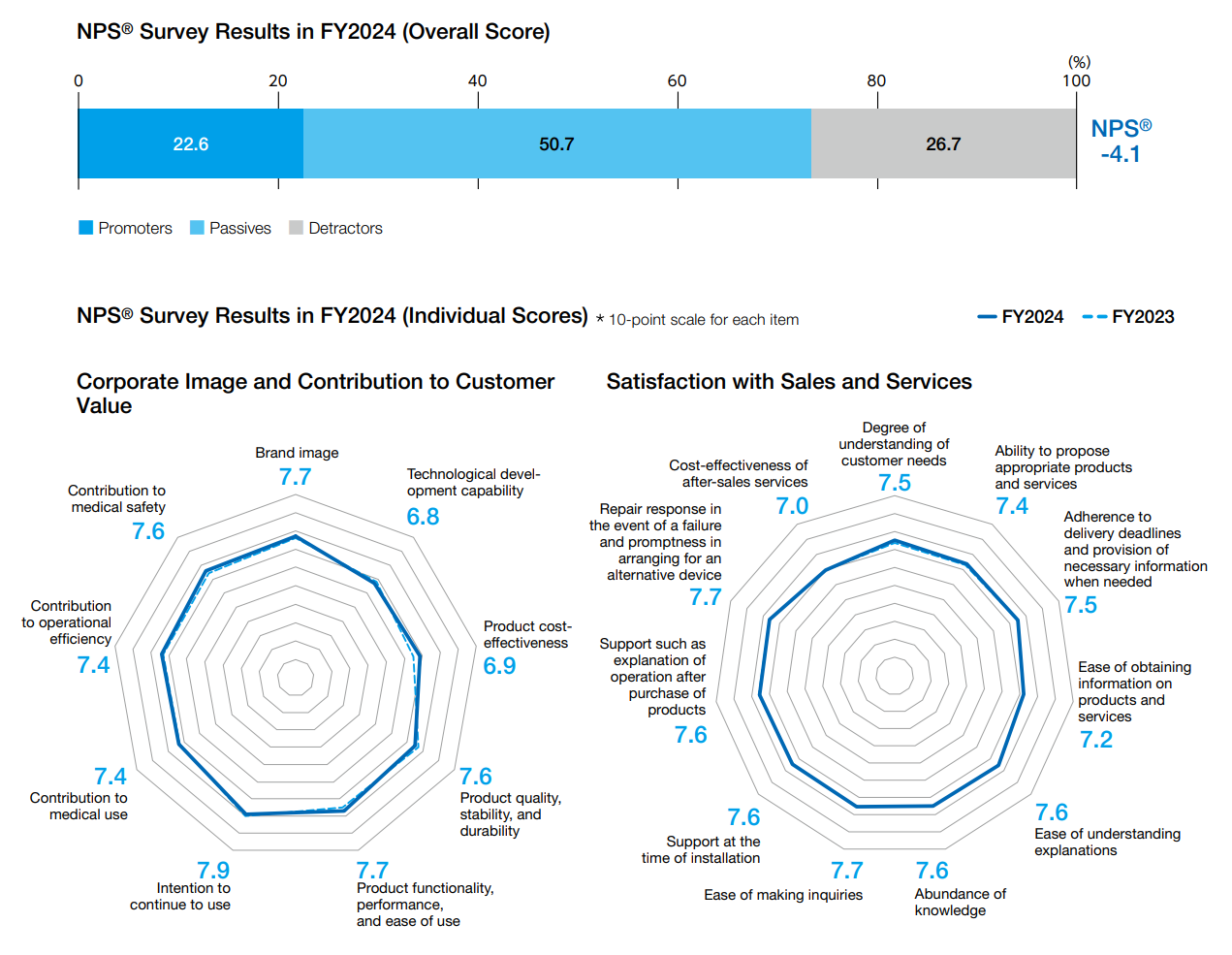
- The NPS® survey is conducted to quantify the degree of attachment to and trust in a company or brand, which has traditionally been difficult to measure, in order to evaluate the customer’s experience at the point of contact with the company and apply it to improvements through future business activities. Since the NPS® survey has a high correlation with business growth rates, it is used by listed companies in the U.S. and Europe and is attracting attention in Japan as a new indicator alongside customer satisfaction.
NPS® is calculated by the following method.
Customers were asked to rate the service on a 10-point scale, with 9 to 10 being “promoters,” 7 to 8 being “passives,” and 0 to 6 being “detractors.” The percentage of promoters (%) to the total number of respondents was subtracted from the percentage of detractors (%), and the resulting number is the NPS value, which is expressed between -100 and +100.
NPS® is a registered trademark of Bain & Company, Fred Reichheld, and Satmetrix Systems (now NICE).
Net Promoter System, Bain & Company’s Website
https://www.bain.com/consulting-services/customer-strategy-and-marketing/customer-loyalty/
