Nihon Kohden has developed and provided Environmentally Friendly Products that take the global environment into consideration as one of our efforts to contribute to society through our business activities. In March 2024, to further strengthen our initiatives, the Company reviewed its standards for assessing environmental impact throughout the lifecycle of its products and services, and started to certify its products and services that meet the standards with the Green Product Label (ISO 14021 Type II environmental labelling for self-declared environmental claims). The Green Product Label certifies the Company’s products and services that meet its standards based on a four-tier assessment ranking in terms of contribution to reducing environmental impact such as CO2 emissions compared to existing products and services, following the implementation of LCA* and environmental assessments, as well as a review and approval process.
*LCA (Life Cycle Assessment) is a method for quantitatively assessing the environmental impact of products or services during their entire lifecycle, from raw material extraction and processing to manufacturing, distribution, usage and disposal of products.

The certification of the Green Product Label was discussed among multiple departments within the Company from the planning stage and released to the public after a review and approval process.

The Green Product Label Certified Products as of May 2025 is as follows.
These products have been labeled based on the Life Cycle Assessment (LCA) calculated specifically for conditions in Japan.
Not all of products and technologies on this website are registered in, approved for sale in, or available for purchase in all countries or regions where this website is accessible. Product images are provided for reference only. Please visit the local website for your region for product, service and contact information.

Three stars certificated products
Products and services which are certified as reducing CO2 emissions by 50% or more compared to existing products through the implementation of LCA
|
Product Name |
Assessment Year |
Country |
Assessment Conditions |
Assessment Results |
Bedside monitors, CSM-1000 series, Lifescope G5 (CSM-1501/1502) |
2023 |
Japan |
■Subject to be assessed: bedside monitors’ main units and accessories ■Functions and conditions to be assessed: |
CO2 emission per unit of the CSM-1501 and CSM-1502 are reduced by 63.4% and 56.9%, respectively, compared to the existing model, the CSM-1901. 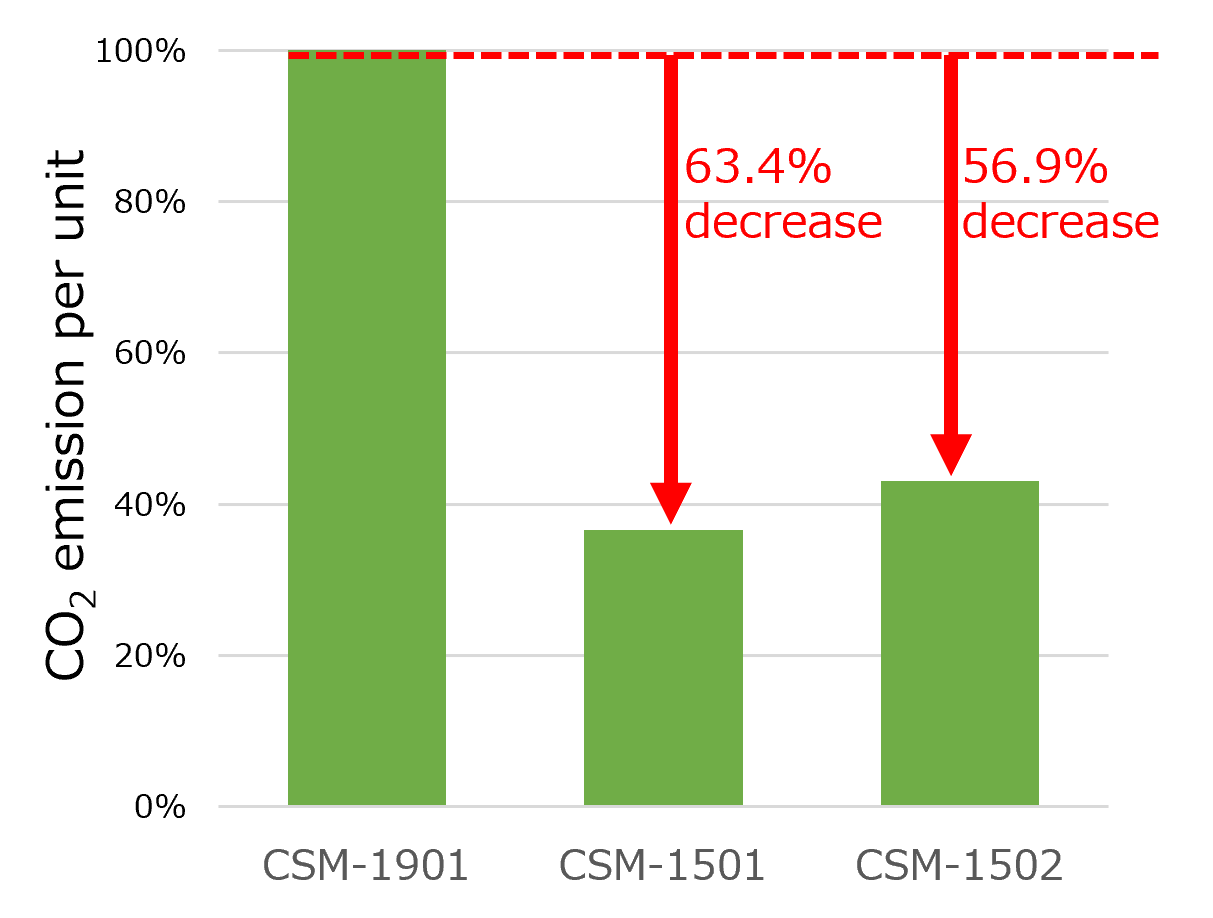
|
Bedside monitors, CSM-1000 series, Lifescope G7 (CSM-1701/1702) |
2025 |
Japan |
■Subject to be assessed: bedside monitors’ main units and accessories ■Functions and conditions to be assessed: |
CO2 emission per unit of the CSM-1701 and CSM-1702 are reduced by 62.3% and 53.7%, respectively, compared to the existing model, the CSM-1901. 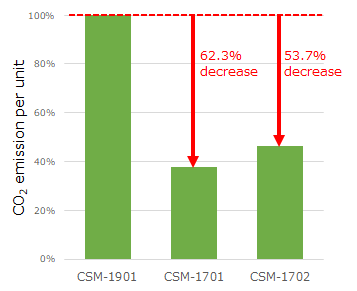
|

Two stars certificated products
Products and services which are certified as reducing CO2 emissions by 25% or more compared to existing products through the implementation of LCA
|
Product Name |
Assessment Year |
Country |
Assessment Conditions |
Assessment Results |

Automated external defibrillator, AED-3100 series, Cardiolife (AED-3100) |
2024 |
Japan |
■Subject to be assessed: AED's main unit, electrode pads, batteries, and AED Linkage*1 |
CO2 emission per unit of the AED-3100 is reduced by 28.4% compared to the existing model, the AED-2100. 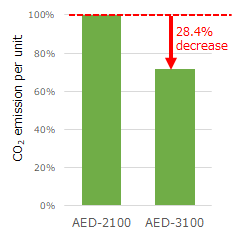
|
Defibrillator, TEC-1000 series, Cardiolife (TEC-1031) |
2024 |
Japan |
■Subject to be assessed: Defibrillator’s main unit, external paddles, and batteries |
CO2 emission per unit of the TEC-1031 is reduced by 29.8% compared to the existing model, the TEC-5631. 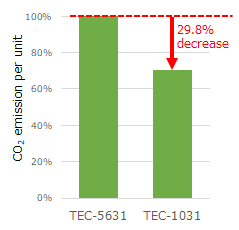
|
*1 AED Linkage (AED Remote Monitoring System) helps support daily maintenance by notifying customers of issues with AED, or the expiration of the AED’s electrode pads and batteries, using an email generated based on information gathered from a self-test performed by the AED unit and sent to a server vial a remote monitoring terminal.
*2 PAD (Public Access Defibrillation) markets include public facilities, schools, and private companies.

No stars certified products b)
Products and services which are certified as having a lower environmental impact compared to existing products based on the Company's internal environmental assessments
|
|
|
Product Name |
Assessment Year |
|
EMG/EP measuring system, MEB-2300 series, Neuropack X3 |
2023 |

Wireless Central Unit, ORG-2100 |
2021 |

Central monitor, CNS-2101 |
2021 |

Mobile AED, AED-M100 series (AED-M100) |
2019 |
Automated external defibrillator, AED-3100 series, Cardiolife (AED-3150) |
2018 |
|
Transmitter, ZS-600P |
2018 |

Bedside monitor, PVM-4000 series (PVM-4731/4733/4751/4753/4761/4763) |
2018 |
EMG/EP measuring system, MEB-9000 series (MEB-9600) |
2018 |

Automated hematology analyzer, MEK-1300 series Celltac α (MEK-1301/1302) |
2018 |
Automated hematology and ESR analyzer, MEK-1303, Celltac α+ |
2018 |

Automated hematology analyzer, MEK-9100, Celltac G |
2016 |

Electrocardiograph, ECG-2400 series (ECG-2450/2460) |
2016 |

Transmitter, ZS-630P |
2015 |
Transmitter, ZS-640P |
2015 |

Polygraphs for cath lab, RMC-5000 |
2014 |
Bedside/transport moitors, BSM-1700 series, Life Scope PT (BSM-1733/1753/1763/1773) |
2013 |

Transmitter, ZS-611P |
2013 |
Defibrillator, TEC-8300 series, Cardiolife (TEC-8321/8322/8332/8342/8352) |
2011 |
